The first-ever guidelines for the product have been released by the Arthritis Foundation. 50 million people in the United States who suffer from arthritis. As the largest organization representing the voice and needs of people with arthritis, the Arthritis Foundation has always welcomed new treatment options because no single drug, supplement or therapy works for everyone. We believe patients should be empowered to find safe management strategies that are appropriate for them. The more options available, the likelier it is that more people will benefit. We are intrigued by the potential of CBD to help people find pain relief and are on record urging the FDA to expedite the study and regulation of these products. the Arthritis Foundation and AARP (American Association of Retired Persons) are finding they must balance the conservative stance of most medical authorities with the real-life needs of their constituents stating, “the tipping point has been reached.”
Animal studies have suggested that CBD has pain-relieving and anti-inflammatory properties, but these effects have not been validated in quality studies in humans. People with arthritis who have tried CBD report noticeable pain relief, sleep improvement and/or anxiety reduction.
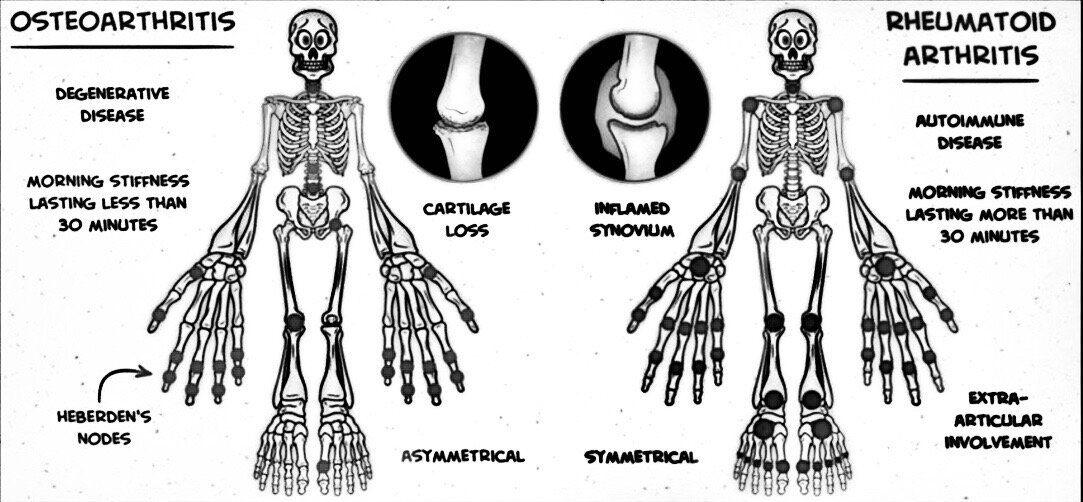
The two most common types of arthritis are:
-
Rheumatoid arthritis (RA): An autoimmune disease in which a person’s immune system attacks their joints, causing inflammation. RA commonly affects the hands and feet and leads to painful, swollen, and stiff joints.
-
Osteoarthritis (OA): A degenerative disease that affects joint cartilage and bones, causing pain and stiffness. It often affects the hip, knee, and thumb joints.
Studies have found that CBD helped to reduce inflammatory pain in rats by affecting the way that pain receptors respond to stimuli. The topical application of CBD had the potential to relieve pain and inflammation associated with arthritis. CB2 receptors are found in unusually high levels in the joint tissue of arthritis patients, making them more sensitive to the effects of cannabinoids like CBD. The use of cannabis is shown to fight inflammation in the joints by activating the pathways of these CB2 receptors.
